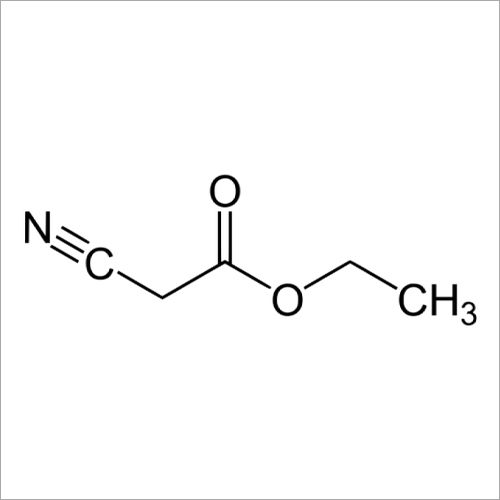
Ethyl Cyanoacetate
100 INR/Kilograms
Product Details:
- CAS No 105-56-6
- Usage Industrial
- Application Pharmaceutical Industry
- Click to View more
X
Ethyl Cyanoacetate Price And Quantity
- 10 Kilograms
- 100 INR/Kilograms
Ethyl Cyanoacetate Product Specifications
- 105-56-6
- Pharmaceutical Industry
- Industrial
Ethyl Cyanoacetate Trade Information
- Cash in Advance (CID)
- 10000 Kilograms Per Week
- 2 Days
- All India
Product Description
Ethyl Cyanoacetate has a molecular weight of approximately 125.13 gm per mole. The compound consists of an ethyl group (C2H5), a cyano group (CN), and a carbonyl group (COCH2) attached to a central carbon atom. It can be synthesized through the reaction of ethyl chloroacetate with sodium cyanide or by the reaction of ethyl acetate with sodium cyanide followed by hydrolysis. It is primarily used as an intermediate & a building block in organic synthesis. Ethyl Cyanoacetate is commonly employed in the production of pharmaceuticals, agrochemicals, dyes, & other speciality chemicals.
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email