X
Tri N Butylamine Price And Quantity
- 120 INR/Kilograms
- 10 Kilograms
Tri N Butylamine Product Specifications
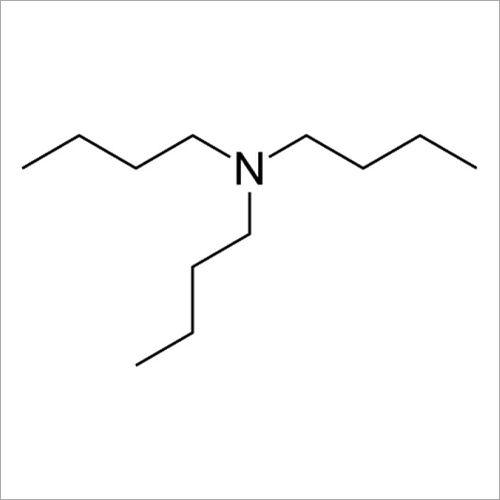
- 102-82-9
Tri N Butylamine Trade Information
- Cash in Advance (CID)
- 10000 Kilograms Per Week
- 2 Days
- All India
Product Description
Tri N Butylamine has a molecular weight of approximately 227.49 gm per mole. The compound consists of three n-butyl groups (C4H9) attached to a central nitrogen atom. It can be synthesized through the reaction of n-butyl chloride with ammonia or by the reaction of n-butanol with ammonia in the presence of a catalyst. It is important to handle tri-n-butylamine with caution as it is a flammable substance. Tri N Butylamine is commonly used in the production of pharmaceuticals, rubber accelerators, corrosion inhibitors, surfactants, & catalysts.
Enter Buying Requirement Details