X
Methanesulfonyl Chloride Price And Quantity
- 10 Kilograms
- 50 INR/Kilograms
Methanesulfonyl Chloride Product Specifications
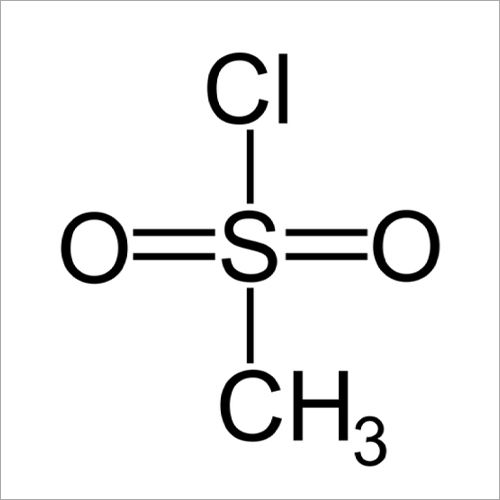
- 124-63-0
Methanesulfonyl Chloride Trade Information
- Cash in Advance (CID)
- 10000 Kilograms Per Week
- 2 Days
- All India
Product Description
Methanesulfonyl Chloride has a molecular weight of approx 114.56 gm per mole. The compound consists of a methyl group (CH3), a sulfonyl group (SO2), & a chlorine atom (Cl) attached to a central carbon atom. It can be synthesized through the reaction of methane sulfonic acid with thionyl chloride or phosphorus pentachloride. It is used as a versatile reagent in organic synthesis. Methanesulfonyl Chloride serves as a source of the methanesulfonyl group & is used for the manufacturing of pharmaceuticals, agrochemicals, dyes, & polymers.
Enter Buying Requirement Details